|  |
|
IS THIS STUFF SAFE TO EAT? How Foods from Biotech Crops are Evaluated for Human Safety in the United States Page 2 Dr. Robert K. D. Peterson
Agricultural & Biological Risk Assessment
Montana State University
bpeterson@montana.edu |  |
At the heart of FDA’s assessment is the concept of “substantial equivalence.” FDA concludes that food derived from biotech crops is substantially equivalent to foods derived from conventional crops when no changes in the food are detected, as discussed above. The new protein may be given GRAS status. Typically, FDA evaluates the biotech crop for 3 to 6 months and issues a letter of agreement with the findings of the developer or marketer of the product if there are no concerns. FDA's review process is evolving and now, primarily to increase public confidence in the regulatory process, the agency soon may require developers to notify it at least 4 months before the commercialization of any biotech ingredients for food and animal feed.
Many people ask why these biotech foods are not labeled by FDA if they contain a new protein that is intentionally added to food (e.g., the Bt protein in corn) or if the food was produced from crops produced using biotechnology. Food additives are defined by FDA as substances intentionally added to foods that are significantly different in structure, function, or amount than current food substances. Also, FDA requires labeling of food only when there is modified nutritional profile or digestibility of food, changes in composition or creation of a new entity, or introduction of a natural plant toxin or non-GRAS food additive. In the case of Bt corn, a new protein was added to corn, but the corn and food derived from it did not require labeling because there was no change in nutritional profile or composition.
Strengths and Weaknesses of Food Safety Assessment for Biotech Crops
In general, the assessment process in the United States for food and crops produced using biotechnology is very robust. These products are evaluated much more thoroughly than any other food or crop. However, foods and crops produced using other processes are rarely assessed for safety (see below). I believe the assessments that are in place today will identify unacceptable food risks posed by biotech crops. But that does not mean that there isn’t room for improvements in the assessments. Indeed, I would argue that some of the improvements are needed to more accurately and comprehensively assess the food risks from these crops. These improvements include: (1) developing a holistic framework for food safety [see below], (2) developing a better definition for substantial equivalence, (3) developing better testing protocols for allergenicity and chronic toxicity, including animal testing models, (4) determining the presence of transgenic proteins in food products, and; (5) determining the fate of ingested transgenic proteins in humans.
|
The Paradox of Biotech Regulation in the United States
Although most recognize that the crop and food should be regulated regardless of the process used to produce it, the reality is that biotech crops (specifically crops produced using recombinant DNA technology) are regulated in the United States because of the way they are produced (Fig. 1). For example, a plant-incorporated protectant derived from recombinant DNA technology, such as Bt corn, is regulated much differently than a plant-incorporated protectant derived from conventional breeding, such as corn resistant to European corn borer that contains the toxin, DIMBOA. Conventionally-bred crops do not undergo any of the EPA, FDA, or USDA regulatory assessments and safety studies that biotech crops undergo. Also, crops derived from other forms of biotechnology, such as chemical and radiation mutagenesis are not regulated. We all consume foods from crops produced from mutagenesis everyday. These crops are produced by bombarding plant tissue with chemicals or radiation, which mutates gene segments, and is much less precise than recombinant DNA technology. Most of the mutants are not desirable, but some result in advantageous properties. | Fig. 1 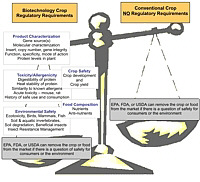
Regulatory requirements for biotech crops (specifically crops derived using recombinant DNA technology) before and after introduction to the market. Conventional crops (including those produced using other forms of biotechnology) have no pre-market regulatory requirements.
|
I believe the root of the problem in regulatory decision-making regarding food risks associated with biotech crops is that there is no comparable regulatory framework for non-engineered crops. In other words, people are asking questions about foods that have never been asked before. But, and here's the rub: the safety and risk issues apply to all new foods and crops, regardless of the technology used to create them. There are thousands of new cultivars for the major crops produced each year. Unless they are produced using recombinant DNA technology, they are not subject to regulatory assessments and approvals in the United States. By regulating based on process instead of final product, we potentially are over-regulating some products and under-regulating others. There are several examples of conventionally-produced crop cultivars in the United States that were great at killing or deterring pests, but they also had acutely toxic levels of inherent plant toxins in them (e.g., potatoes (toxin = glycoalkaloids) and celery (toxin = furocumarins). These new crops cultivars were not evaluated by US regulatory agencies. In the US (and much of the world), there is no holistic framework for evaluating and regulating food safety. Food risk needs to be addressed, but it needs to be much broader than biotech crops.
In Canada, all novel foods, regardless of the technique used to produce them, are subject to regulatory approval. This, I believe, is the logical and appropriate framework we should use in the United States. However, it is not without its drawbacks and costs. As a scientist and risk assessor, I would argue that all foods need to undergo similar risk assessments. However, as a society, how can we afford to add billions of dollars of cost to our food? These questions extend well beyond technical and scientific issues and require answers from all levels of society.
Suggested Readings
U.S. Regulatory Oversight in Biotechnology,
http://www.aphis.usda.gov/biotech/OECD/usregs.htm
Council for Agricultural Science and Technology [CAST]. 2001. Evaluation of the U.S. regulatory process for crops developed through biotechnology. Issue Paper 19, 14 pp.
 |











